Inhibitors of the arginine-vasopressin (AVP) V2 receptor (V2R) are key therapeutic compounds for treating hyponatremia consecutive to congestive heart failure, hypertension or hepatic cirrhosis, but also for treating polycystic kidney diseases. Today only two molecules are on the market, Tolvaptan (TVP) and Conivaptan. While TVP is selective to V2R, chronic treatment leads to side effects, mainly liver toxicity. Conivaptan, used for treating hyponatremia in hospitalized patients with underlying heart failure, is unfortunately not selective for V2R displaying a high affinity for the related V1a vasopressin receptor.
Finding new compounds with better selectivity and less side effects is crucial. To that purpose, rational drug design based on experimental G protein-coupled receptor structures is a powerful avenue to develop better drugs. So far, the lack of inhibitor-bound V2R structures has impaired this strategy.
After solving the active structures of the AVP-bound V2R-Gs protein and AVP-bound V2R-barrestin1 complexes, the team “Membrane Protein Structure and Function for Drug Discovery” headed by Bernard Mouillac and Sébastien Granier at the IGF, in collaboration with Gunnar Schulte and Julien Bous at the Karolinska Institute in Stockholm, Nicolas Gilles at the CEA in Paris and Charline Mary at the Center of Structural Biology (CBS) in Montpellier, determined using cryo-electron microscopy the near atomic structures of the receptor in complex with two inhibitors, the therapeutic compound Tolvaptan and the green mamba snake mambaquaretin toxin (MQ1). Both ligands bind into the orthosteric binding site but with substantial differences. The small molecule TVP binds deeper than MQ1, and blocks V2R through a direct contact with the toggle switch residue W284 in the transmembrane domain 6. The Kunitz-fold toxin displays extensive contacts with extracellular and transmembrane residues and inhibits the receptor in a specific manner. As anticipated from TVP and MQ1 pharmacological properties, both structures represent inactive V2R conformations. Their comparison with those of the active AVP-bound V2R reveals the molecular mechanisms modulating receptor activity
These results constitute the first inhibitor-bound V2R structures ever described. Their comparison with active V2R structures (in the presence of agonists and signaling partners) will guide the rational development of new molecules leading to improved therapies. This research perspective is crucial when dealing with diseases that are difficult to manage and overwhelming for patients. The mini-protein MQ1-bound V2R structure opens a new pharmacology approach for treating water homeostasis and renal diseases.
This work has just been published in Nature Communications.
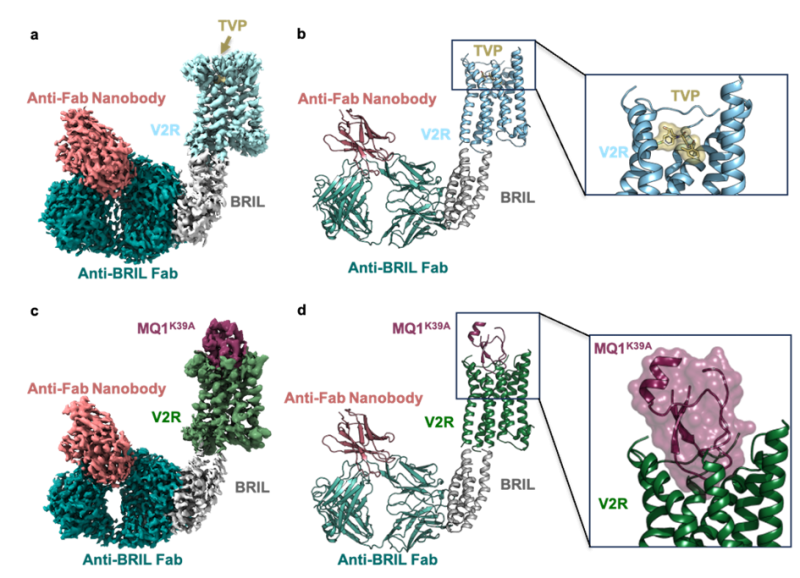
Inactive structures of V2R in complex with tolvaptan and mambaquaretin 1 (MQ1).
In a and c, cryo-electron microscopy density maps are shown. In b and d, corresponding 3D models. A close-up view of the TVP-V2R and MQ1(K39A mutant)-V2R complexes is shown on the right. The BRIL module, the anti-BRIL antibody fragment (Fab) and the anti-Fab nanobody are used as scaffolding partners.


