Membrane receptors are essential for cell-to-cell communication, making them a major target for drugs. Understanding how they function, and their dynamics both structurally and within the cell, is essential for the development of new drugs. Today, these studies are almost always carried out on recombinant receptors, expressed in heterologous cells. However, it is now recognized that the properties of receptors can vary greatly depending on their environment, their density on the cell surface, the accessory proteins with which they assemble, and their location in cellular sub-compartments, both on the surface and in intracellular organelles. It is therefore essential to be able to study native receptors in their natural environment: in the cells that naturally express them. An essential approach to monitoring and studying these receptors, measuring ligand binding, tracking their localization and movement within the cell, requires their labeling. While the use of labeled antibodies may offer advantages, their reversible binding, and the size of the antibodies may largely limit their use.
Within the framework of the ANR Lanthslider contract involving two IGF teams, the team “Neuroreceptors, dynamics and functions” headed by Philippe Rondard and the team “Pathophysiology of synaptic transmission” headed by Julie Perroy, as well as the chemistry laboratory headed by Amadeu Llebaria in Barcelona, and our colleagues at Revvity/Cisbio, an original approach to covalent labeling of a membrane receptor has been set up and validated. This approach involves the synthesis of a compound comprising three functional entities: a high-affinity ligand for the receptor, enabling the compound to be targeted specifically to the receptor, a reactive group that can form a covalent bond with a lysine residue of the receptor, and a fluorophore. A key feature of the chemical reaction with lysine is the breaking of the bond with the high-affinity ligand, so that once the fluorophore is attached to the receptor, the ligand is released, leaving the receptor activation site intact and accessible.
This work was successfully carried out by Xavier Gomez-Santacana, a post-doc in Rondard’s team, in conjunction with chemists from Barcelona and Revvity, enabling the selection of the best chemical tool for labelling the dopamine D1 receptor. Microscopic observations by Marin Boutonnet (postdoc in Perroy’s team) were used to optimize the protocol. Finally, Enora Moutin (researcher in Perroy’s team) and Marta Cimadevila (postdoc in Rondard’s team) demonstrated that the labelled receptor is still functional.
This work has just been published in the journal Communications Chemistry.
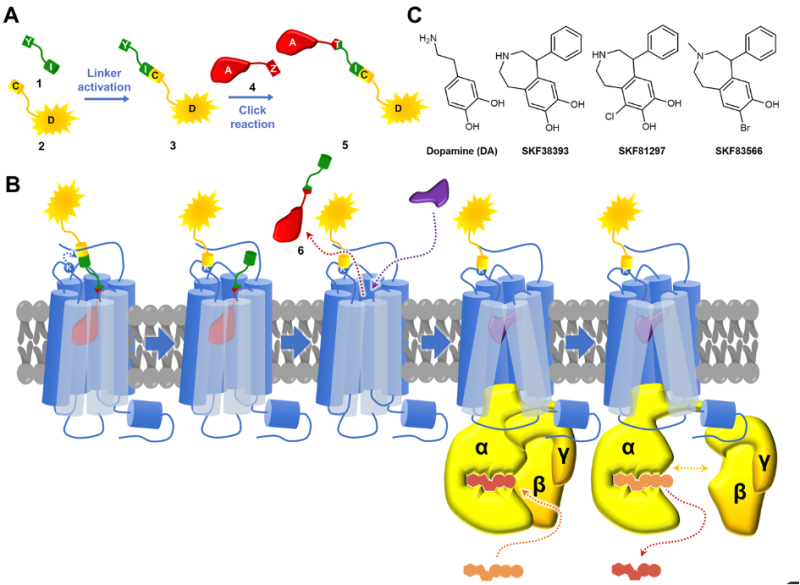
Schematic diagram of the covalent receptor labelling method. A) schematic diagram of the labelling tool, comprising a fluorophore linked via an arm to a reactive group (yellow), associated with a high-affinity ligand (red) via a linker (green). B) following binding of the ligand to the receptor, the reaction takes place with a lysine residue, allowing covalent attachment of the fluorophore to the receptor, releasing the affinity ligand. This frees the activation site. C) Ligands that can be used to label D1 receptors.


